Abstract
Introduction: Betrixaban is a factor Xa inhibitor indicated for the prophylaxis of venous thromboembolism (VTE) in adult patients hospitalized for an acute medical illness. A physiologically based pharmacokinetic (PBPK) model for betrixaban provided the mechanistic understanding of the contribution of P-glycoprotein (P-gp), as well as CYP3A4, to the disposition of betrixaban, and predictions of the effects of drug-drug interactions mediated by P-gp and/or CYP3A4. The PBPK model specifically evaluated contribution of the interaction with the P-gp transporter to the disposition of betrixaban, as well as the effects of co-administration of P-gp inhibitors (ketoconazole, verapamil, clarithromycin) on betrixaban pharmacokinetics (PK). Further, in vitro and clinical studies confirmed the lack of a significant interaction of betrixaban with CYP3A4.
Methods: The base PBPK model was developed using physiochemical data, in vitro metabolism andtransport data generated using recombinant CYP enzymes and human hepatocytes, and relevant clinical data (eg, observed renal clearance). The model was verified by comparing model predictions with PK data derived from single oral doses of betrixaban (from 80 to 550 mg) in healthy volunteers. The combined contribution of intestinal and hepatic P-gp transport to the disposition of betrixaban was verified by comparing model predictions to the observed ketoconazole and verapamil clinical drug-drug interaction data from healthy volunteers. The verified PBPK model was prospectively employed to predict the impact of a strong P-gp inhibitor (clarithromycin) and a moderate P-gp inhibitor (diltiazem) on betrixaban PK, and to predict the impact of food intake on betrixaban PK and compare it against the observed data in phase 3 studies. The Simcyp Population-Based Simulator (v15 release 1) was used for all simulations.
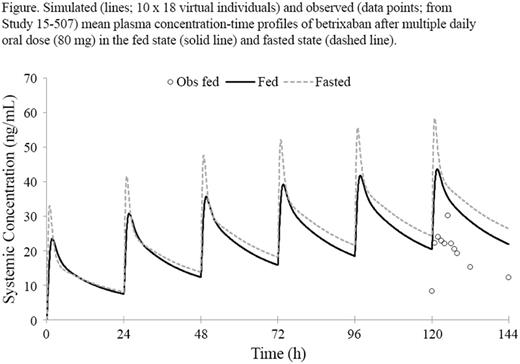
Results: The major clearance pathway for betrixaban was biliary clearance (assigned to hepatic P-gp) as scaled from uptake data generated in human hepatocytes, accounting for ~80% of clearance after IV administration (CLIV). CYP3A4 played virtually no role in the disposition of betrixaban. The base PBPK model incorporating an intestinal P-gp component within the ADAM model resulted in simulations that predicted concentrations very close to the clinically observed pharmacokinetic non-linearity arising from multiple oral dosing of betrixaban in the dose range of 80 to 550 mg in healthy volunteers. The model predicted that the inhibition of intestinal and hepatic P-gp transport of betrixaban would result in the geometric mean area under the curve (AUC) and maximal concentration (Cmax) ratios due to concurrent ketoconazole treatment of 2.4 and 2.5, respectively, which were consistent with the observed ratios of 2.1 and 2.3. The predicted betrixaban geometric mean AUC and Cmax ratios due to concurrent verapamil treatment were 2.3 and 3.1, respectively, compared with the observed ratios of 3.0 and 4.7. The model qualitatively predicted the negative impact of food intake on betrixaban exposure (Figure). The predicted AUC and Cmax for patients treated with betrixaban in the fed state in the phase 3 study were within 1.5-fold of the observed values. In the final application of the PBPK model to predict the impact of the strong and moderate P-gp inhibitors, the predicted AUC and Cmax ratios due to clarithromycin treatment were 3.76 and 3.11, respectively, and due to diltiazem treatment were 1.1 and 1.1, respectively.
Conclusions: The PBPK model for betrixaban adequately predicted the clinically observed PK non-linearity and accounted for the observed drug-drug interaction effects due to co-administration of betrixaban with ketoconazole and verapamil in healthy volunteers. The PBPK model did not predict a role for CYP3A4 in the clearance of betrixaban and accurately predicted a negative food effect. Intestinal and hepatic P-gp were identified as major contributors to the observed non-linearity in the kinetics of betrixaban and observed drug-drug interaction effects with P-gp inhibitors.
Leeds: Portola Pharmaceuticals, Inc.: Employment, Equity Ownership. Ke: Portola Pharmaceuticals, Inc.: Consultancy. Rowland Yeo: Portola Pharmaceuticals, Inc.: Consultancy. Curnutte: Portola Pharmaceuticals, Inc.: Employment, Equity Ownership. Conley: Portola Pharmaceuticals, Inc.: Employment, Equity Ownership, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal